FORWARDING CLINICAL SUPPLY CHAIN SECURITY STRATEGY WITH BACKWARD INTEGRATION OF STARTING MATERIALS AT CDMOs
Ravikrishna Chebolu, Ph.D., Vice President – Laurus Synthesis
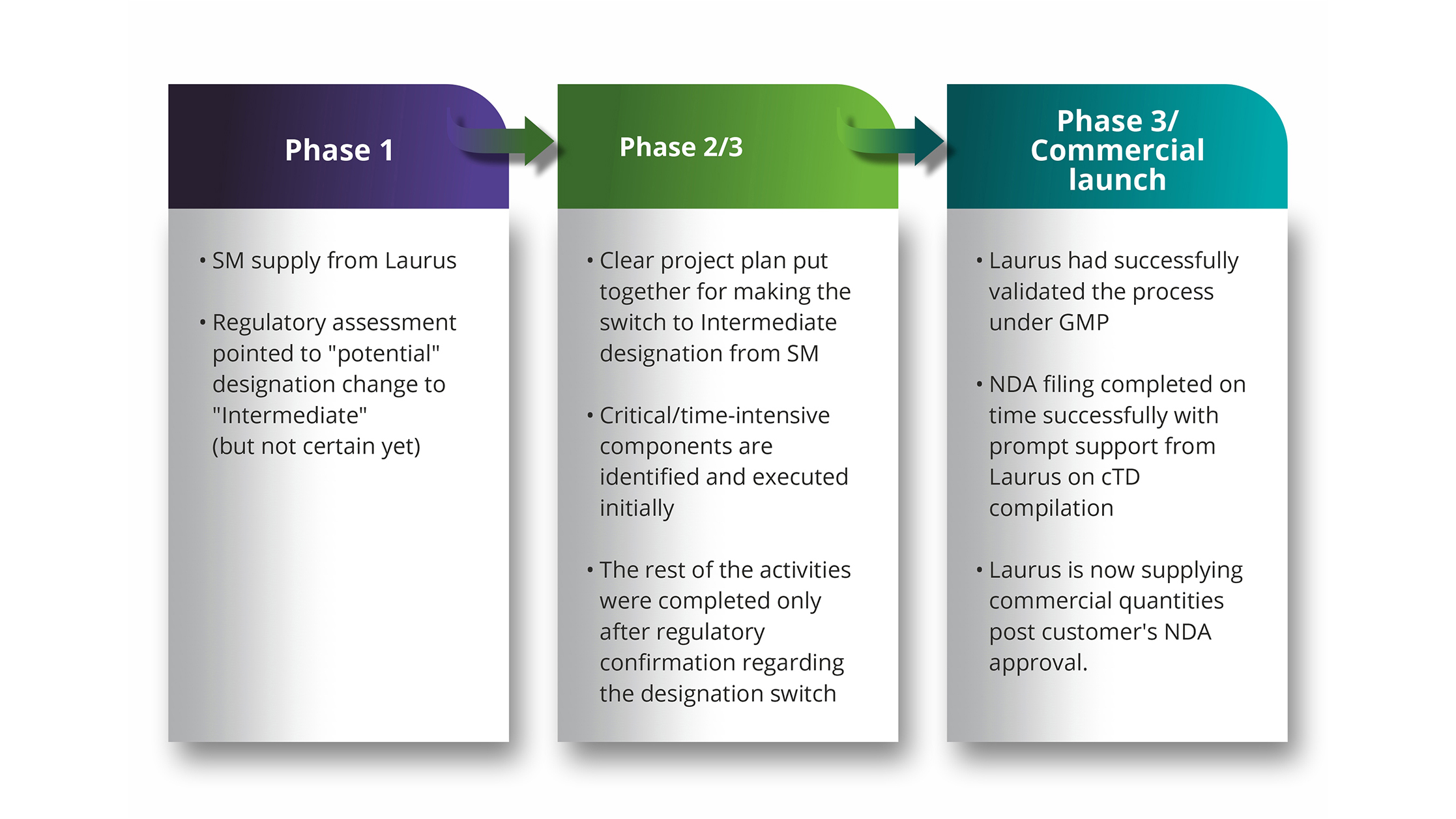
A lot has changed in pharmaceutical supply chain world over last decade and in particular within last two years. We all have seen serious supply chain uncertainties for one reason or other, the most recent being the disruptions worldwide due to COVID-19. It has now become all the more important for pharmaceutical companies and their CDMO partners to have a clear strategy in place, well in advance, to avoid pitfalls in efficient management of clinical supplies. Most CMC managers have experienced that one of the critical pieces in securing drug substance supply chain is the “starting material” (SM) supply. Just when everything else including the scheduling of drug substance and drug product manufacturing seems all set, organizations are often caught completely unprepared to deal with uncertainties in SM supplies in last minute. Another interesting dimension of this challenge is the constantly evolving understanding around what's designated as "starting material" in the route of drug substance synthesis (RoS) as part of regulatory filing. The starting materials of the designated "starting material" in the synthetic route have also assumed much more significance in the current regulatory environment than before.

The critical piece of the puzzle in clinical/commerical supply chain security: “Starting Material”
It is important to understand the drivers for SM backward integration clearly (e.g. quality risk, supply risk, CoGs reduction) by asking the right questions early on in the Clinical Development program
Our experience at Laurus over the last >12 years in supporting the clinical development programs of global pharma/biotech helped us understand that backward integration of starting materials at API/Intermediate manufacturers, where appropriate, can enormously help stabilize the overall supply chain. However, each program requires a slightly different strategy depending on the nature of challenge; and the success of that strategy depends on how clearly the goal of the exercise is identified and defined at the beginning itself. Having understood the severity of the SM supply risks ahead of time, Laurus had invested in creating R&D and manufacturing resources dedicated for the development and commercial manufacture of Starting Materials. This article details a few examples wherein Laurus worked closely with our customers to ask the most important questions, as much as possible early on in project execution, to provide sustainable long term solutions for the sourcing challenges on hand.
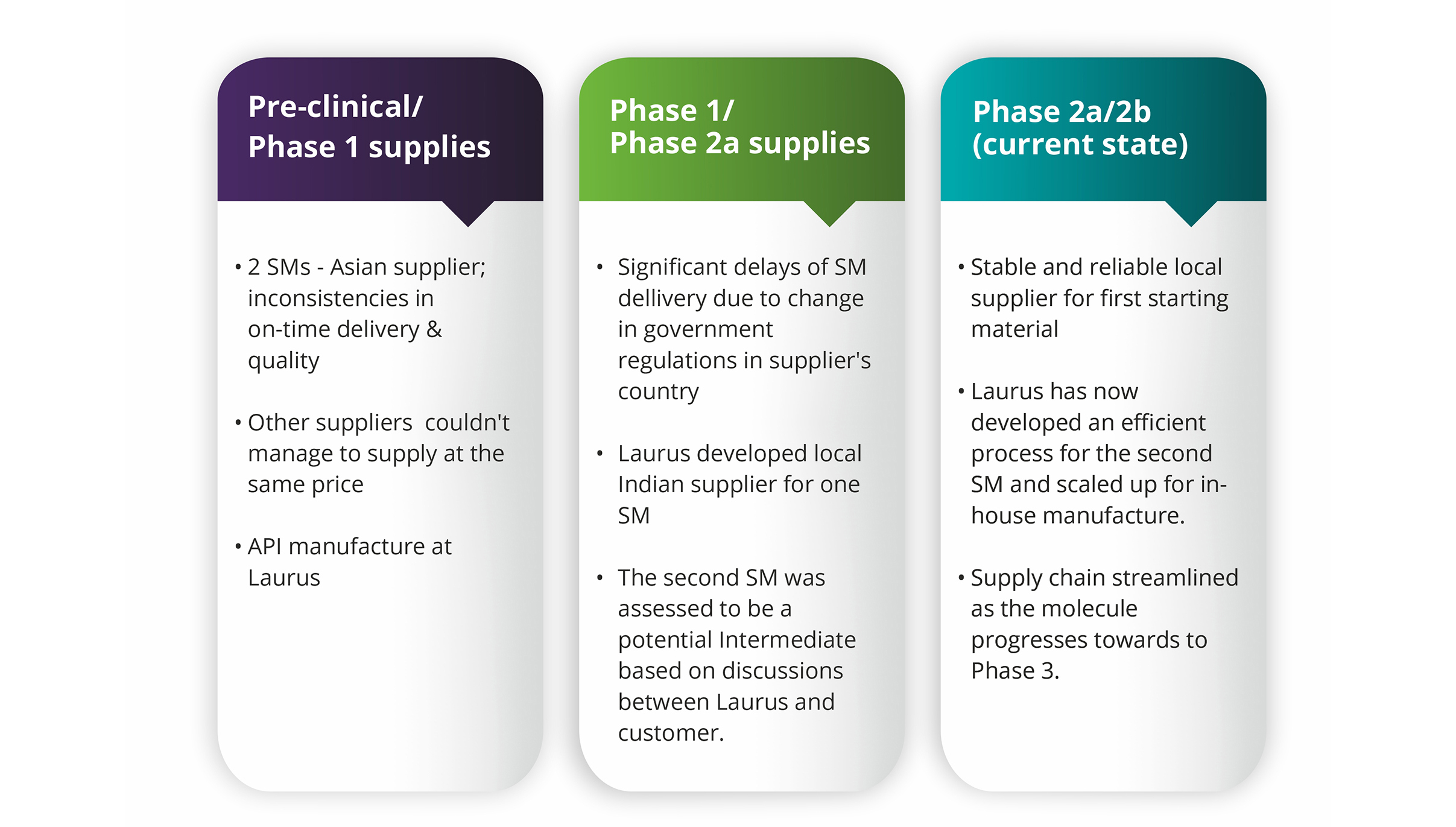
How do we manage risks from our current "single" SM source in the long run?

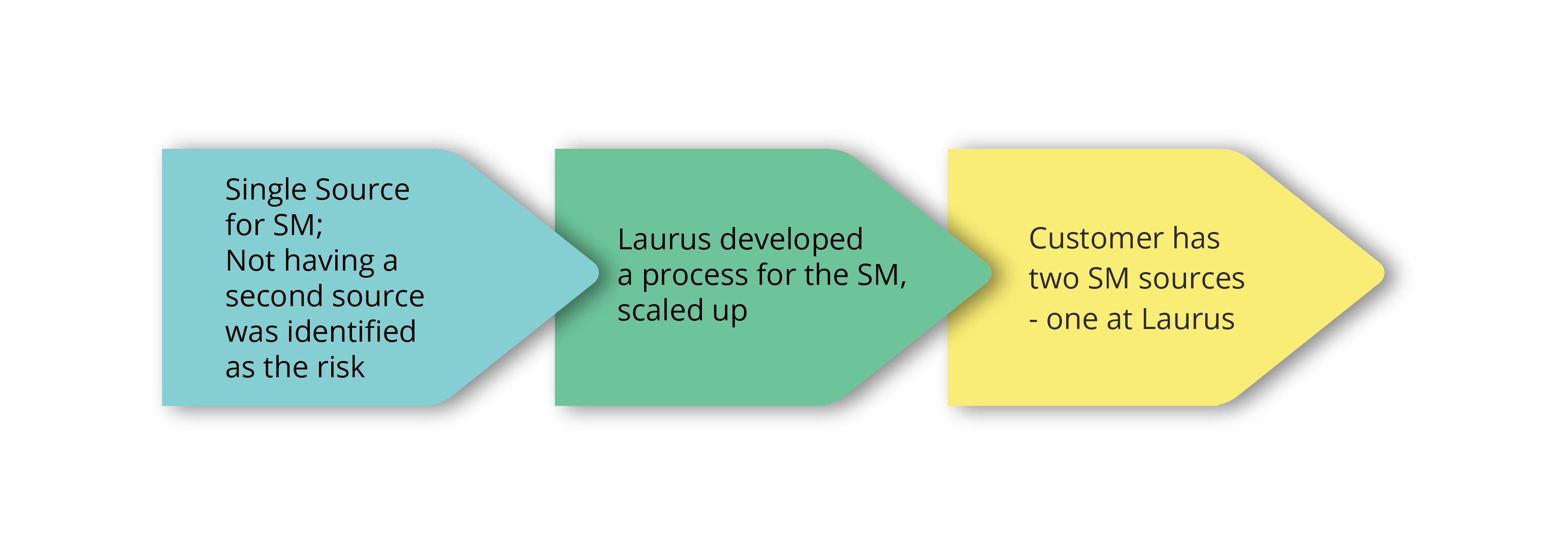

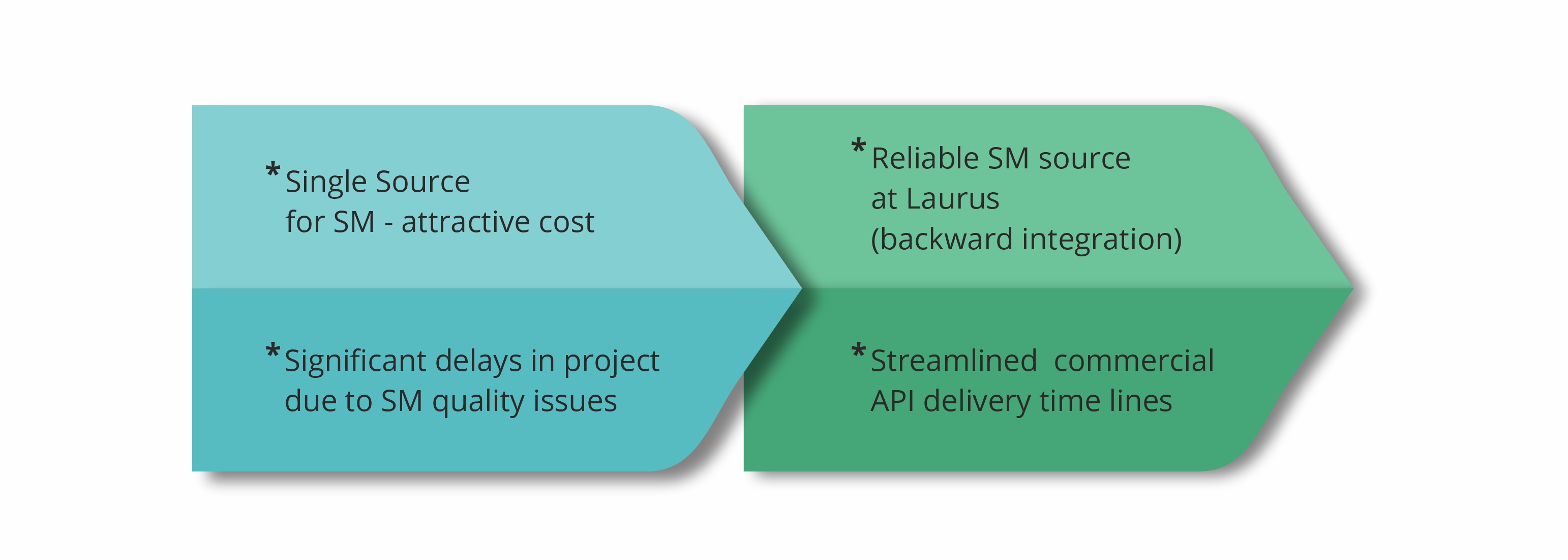
Complexities in importing one of the starting materials from a supplier in Asia (single source) into India was identified as a potential risk factor in the commercial supply of a regulatory intermediate to one of our big pharma customers. For several years, it seemed pretty much risk-free, but over the last two years things have changed significantly in terms of supply assurance due to complex impurity procedures. The SM backward integration strategy was concluded as the only way to mitigate supply risks.

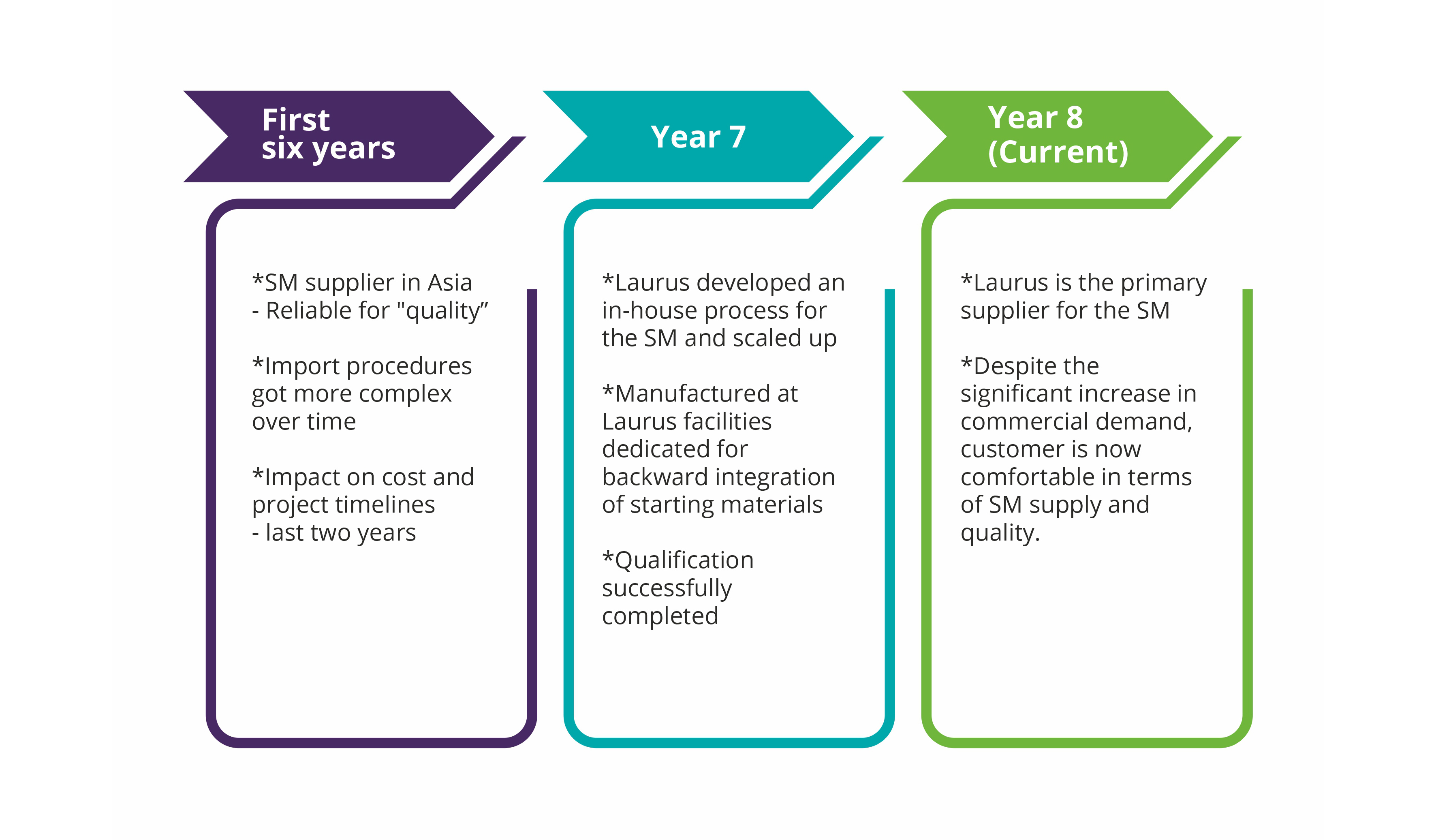
Using the in-house developed process Laurus quickly scaled up and successfully qualified the material by use in production batches of the Intermediate. Since this starting material is required in multi metric ton scale, one of the dedicated starting material manufacturing facilities at Laurus was greatly helpful to manage tight delivery timelines. As the customer demand for the regulatory intermediate is expected to further increase due to API launch in new geographies in the coming years, this SM backward integration exercise has helped to significantly reduce the supply chain uncertainty along with some overall cost efficiencies as well.
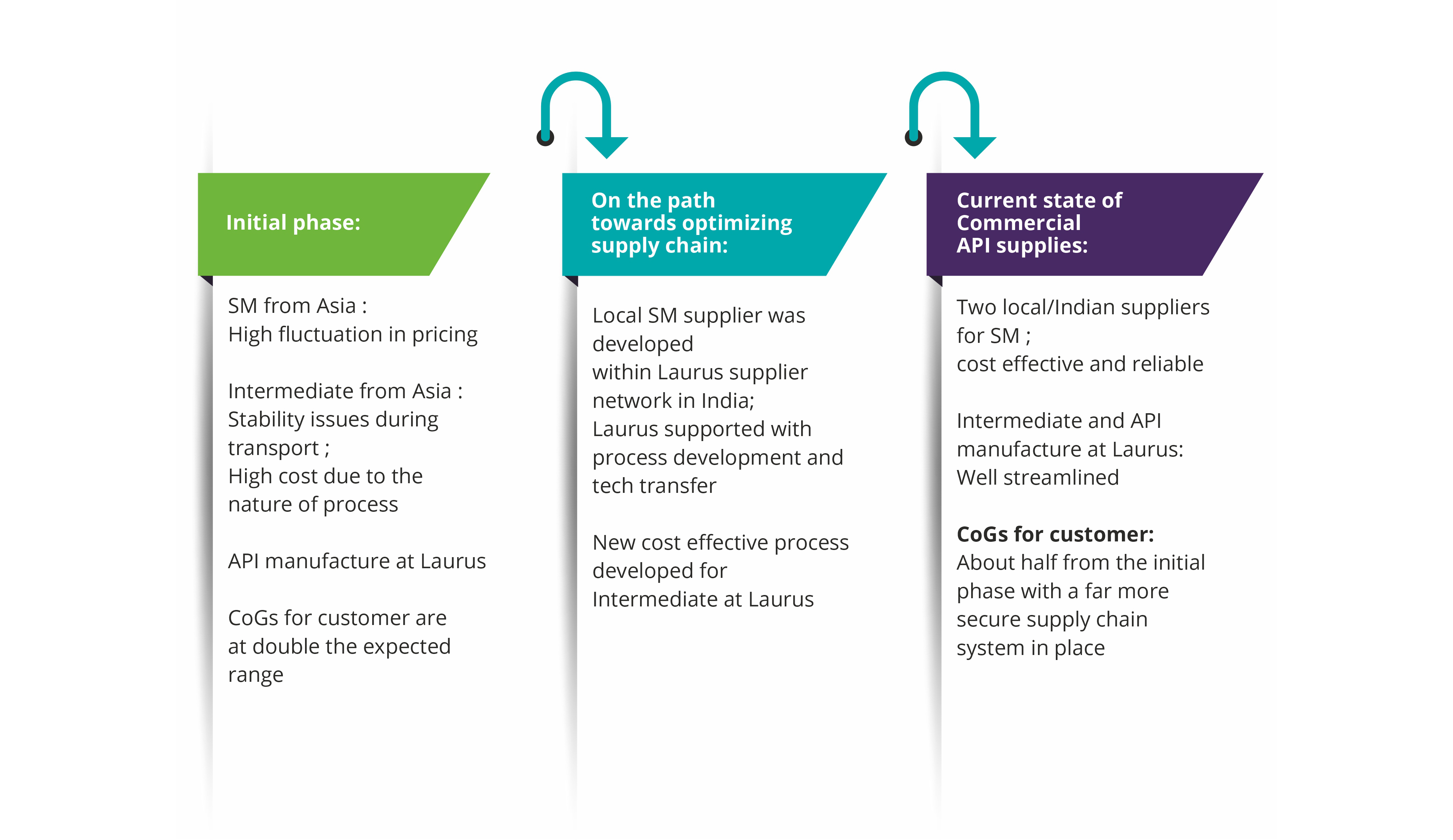
How do we reduce overall Cost of Goods (CoGs) by redesigning the supply network?