The term “Safety” in the Pharmaceutical / Healthcare sector is often only associated with Patient Safety. Delivering Safe and Quality medicines to patients is reliant on maintaining Safety and Quality in manufacturing operations. At Laurus we strive to abide by “Safety first, Quality always”. Although evaluating and prioritizing inherently safe chemistries is a key-objective during process development; Hazardous chemistries can sometimes still be the only viable way to produce certain compounds. Alongside scale-up considerations, to assess the chemical hazards associated with reagents, chemicals and solvents utilized - a thorough process safety evaluation is paramount
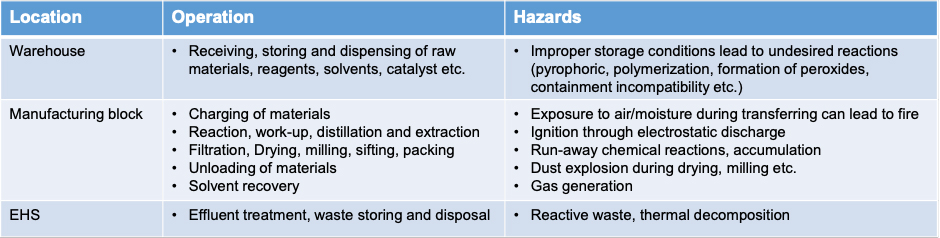
Process safety evaluation is about identifying the process hazards which can lead to catastrophic events such as fire, deflagration, explosion, release of toxic gases etc. and devising mitigation strategies throughout the utilization cycle of materials. Typical operations and the associated risks in drug substance manufacturing include:
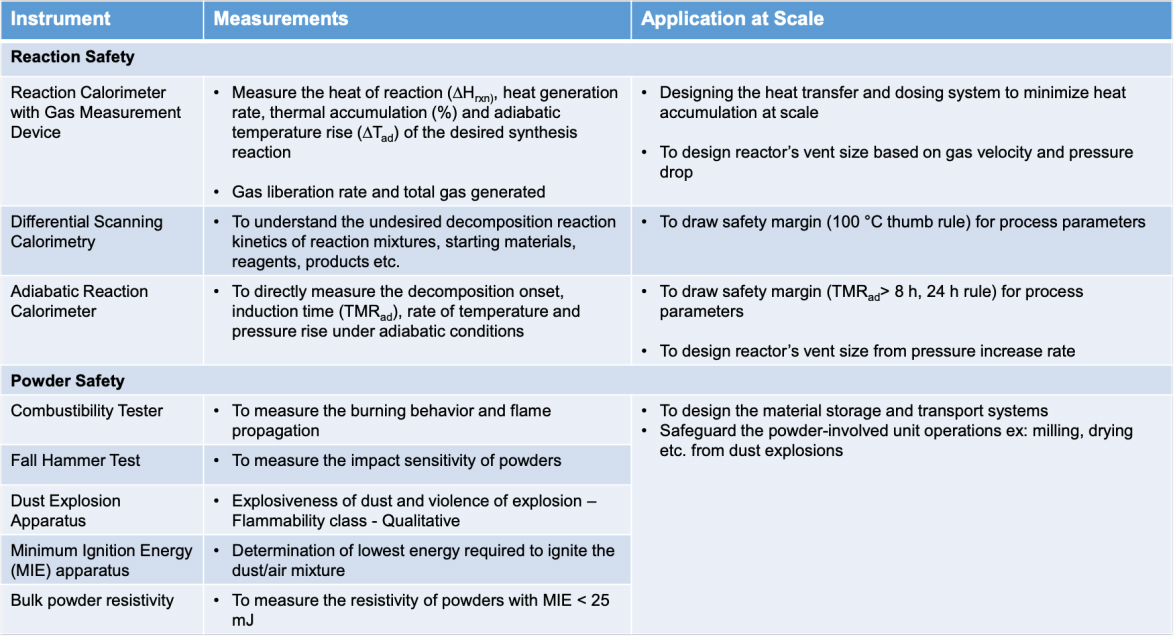
Process Safety Assessments at Laurus are undertaken by dedicated group comprising of Chemical Engineers at our Process Safety and Engineering (PSE) laboratory. PSE laboratory is equipped with various equipment to assess Reaction Safety and Powder Safety towards assessing critical information on the safety aspects of desired synthesis reaction, undesired secondary reactions, powder properties etc.
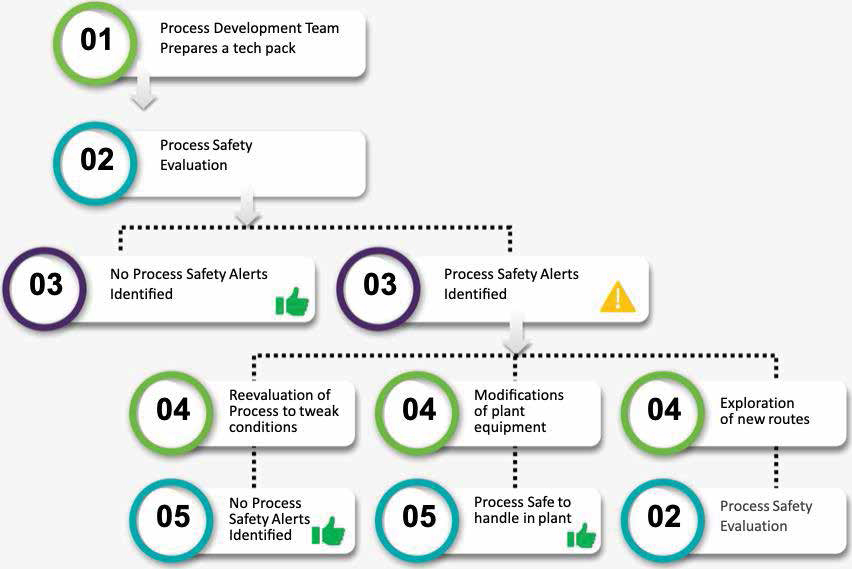
With the aid of these instruments, our PSE team comprehensively evaluates every stage of drug synthesis, and a Basis of Safety document is generated based on the inferences drawn. This document establishes the guidance for technology transfer, HAZOP assessments and manufacturing. A typical process safety workflow looks as shown below:
A nitration reaction involves dissolving the compound in acetic acid and adding 70% aq. Nitric acid to initiate the reaction was studied at Laurus PSE laboratories.
RC1e data suggests that the reaction is slow with 87.5% thermal accumulation at process temperature (Tp) of 40 °C. The adiabatic temperature rise ∆Tad of 61.4 °C was noted. The maximum temperature which the process can achieve in case of cooling failure (MTSR = Tp + ∆Tad) is 101.4 °C.
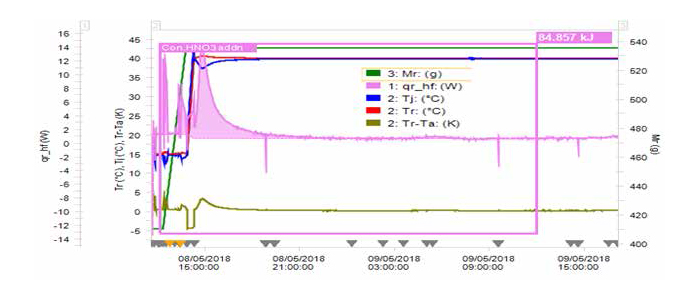
Figure 1 RC1e data of the nitration reaction
Based on DSC data- Post reaction completion, there exists a decomposition reaction which initiates at 88°C with energy liberation of 985.1 J/g. ARC data further reveals that the decomposition initiates at 60.2 °C under thermodynamic conditions and predicts that TMRad (8h) is 54.6 °C and TMRad (24h) is 42.5 °C. As per the Stoessel’s criteria, this reaction falls in class 5 with high criticality index.
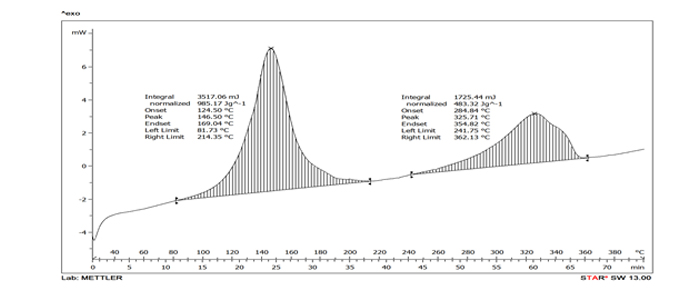
Figure 2 DSC data of after reaction mass
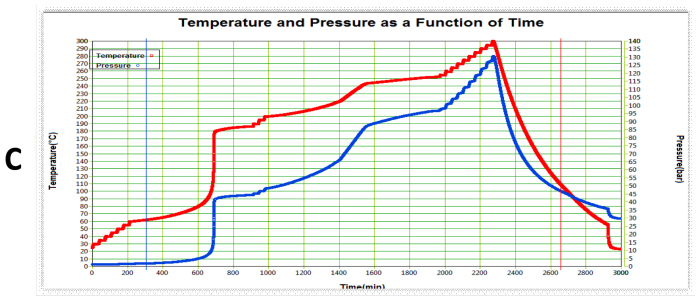
Figure 3 ARC data of after reaction mass
Based on the PSE evaluation, the process was further optimized and additional engineering controls were established at manufacturing facility.
Optimized process included addition of Nitric acid in 2-lots at higher temperature (40 °C instead of 15 °C) thereby reducing the thermal accumulation from 87.5 % to 17.5% and the MTSR from 101.4 °C to 52.3 °C. Optimized reaction conditions can be categorized in criticality class-4 against criticality class-5 originally. On the equipment engineering front, a Two-pronged control strategy was implemented. Firstly, towards achieving controlled addition of Nitric Acid, an orifice-based mass flow controller been used to control the flow rate. Second, automated safety interlocks based on Reaction Mass Temperature and Reactor Jacket temperature differential (Inlet vs outlet) were established. If set-temperatures are met - audible alarms are triggered along with automated reaction quenching, thereby preventing any run-away reaction.

Dr. Satyanarayana Thirunahari is the General Manager at Laurus Labs Limited, Hyderabad, and is responsible for process safety and engineering of active pharmaceutical ingredients, scale up and technology transfer to manufacturing scale. With 13+ years of industry experience, he has published 7+ articles in international journals, has given several well-received talks at national and international conferences, and has 17+ patents to his name.
© Laurus Synthesis. All rights reserved