Why Us
Our development and manufacturing teams maintain a tight focus on performance at scale, continuous process improvement, securing and de-risking supply chains to provide efficient, regulatory compliant, cost-effective and long-term commercial drug substance solutions.
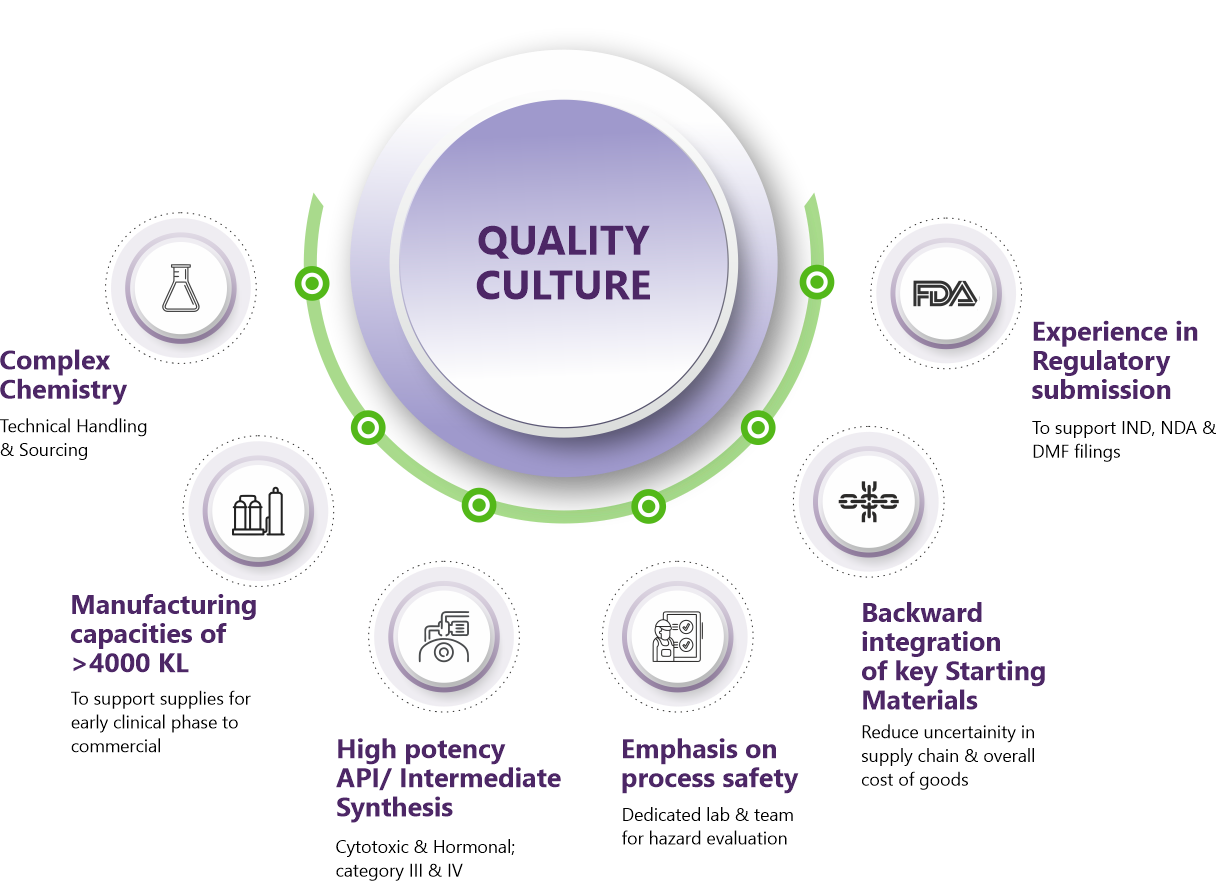
How we helped our partners

Supply of Key Starting Material and Regulatory Intermediate for a North American Biotech
Download Case study

The Path towards success in HPAPI manufacturing – Challenges and Solutions
Download Case study

Managing scope changes in drug development programs at CDMOs: Need for Planning and Flexibility
Download Case study
