Combining efficiency and safety in high-potent drug substance manufacturing
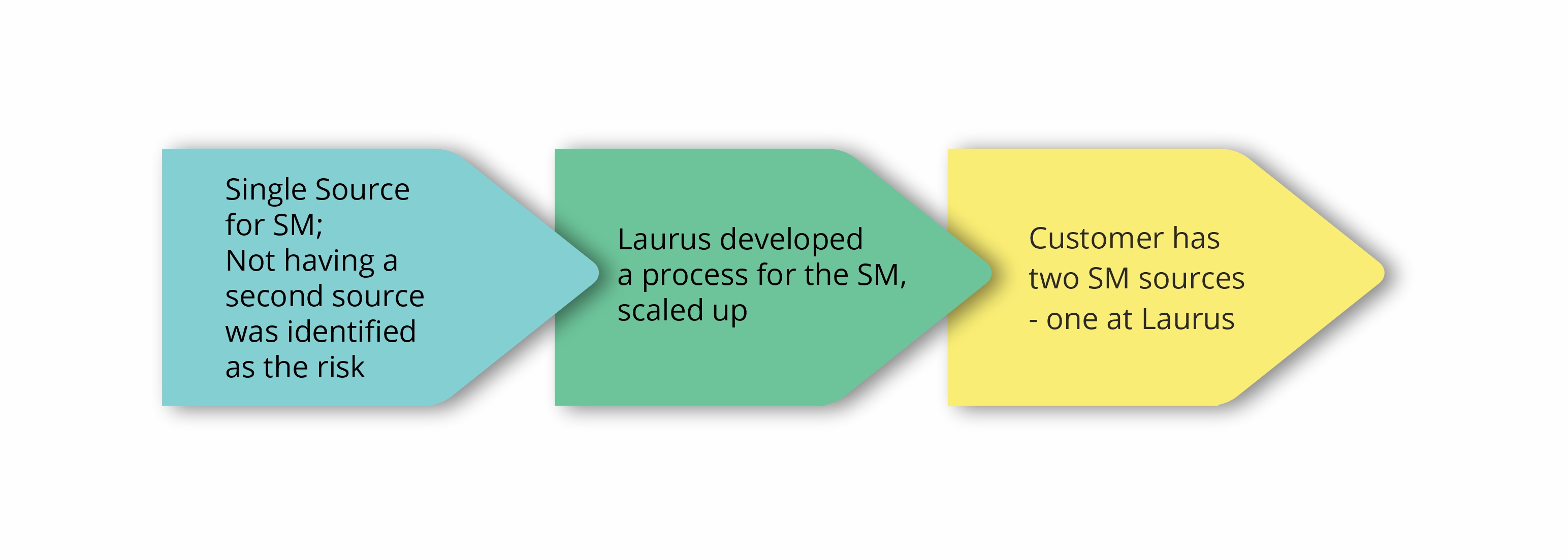
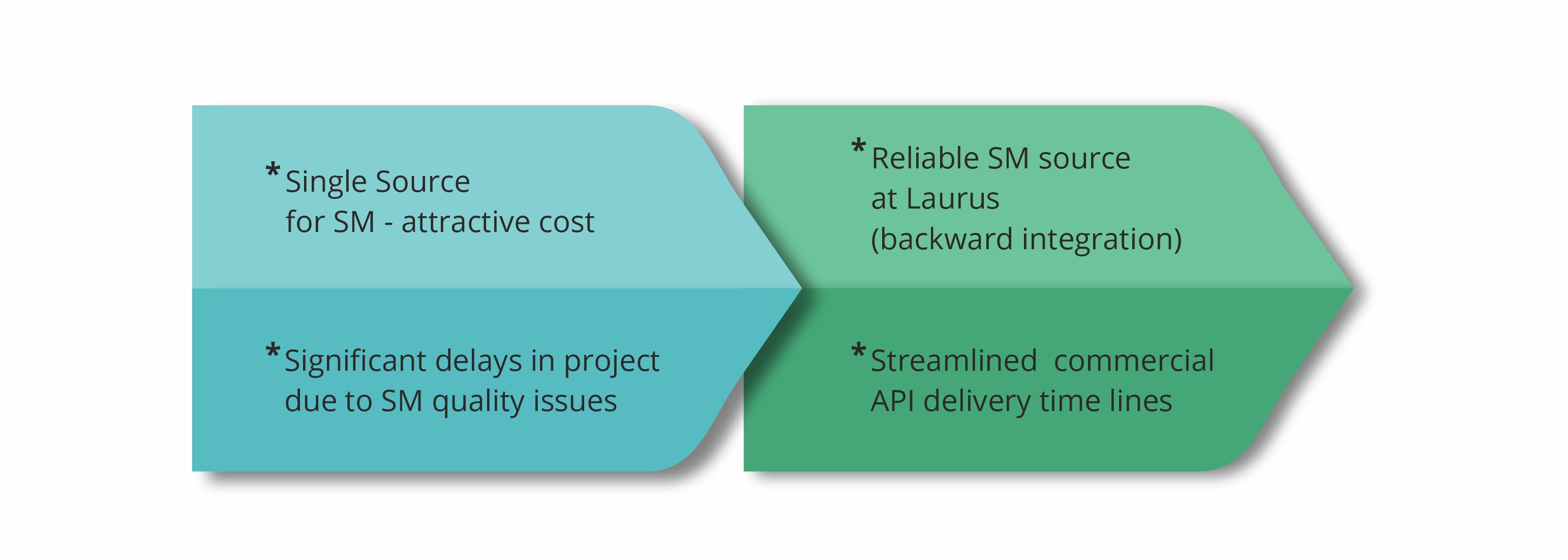
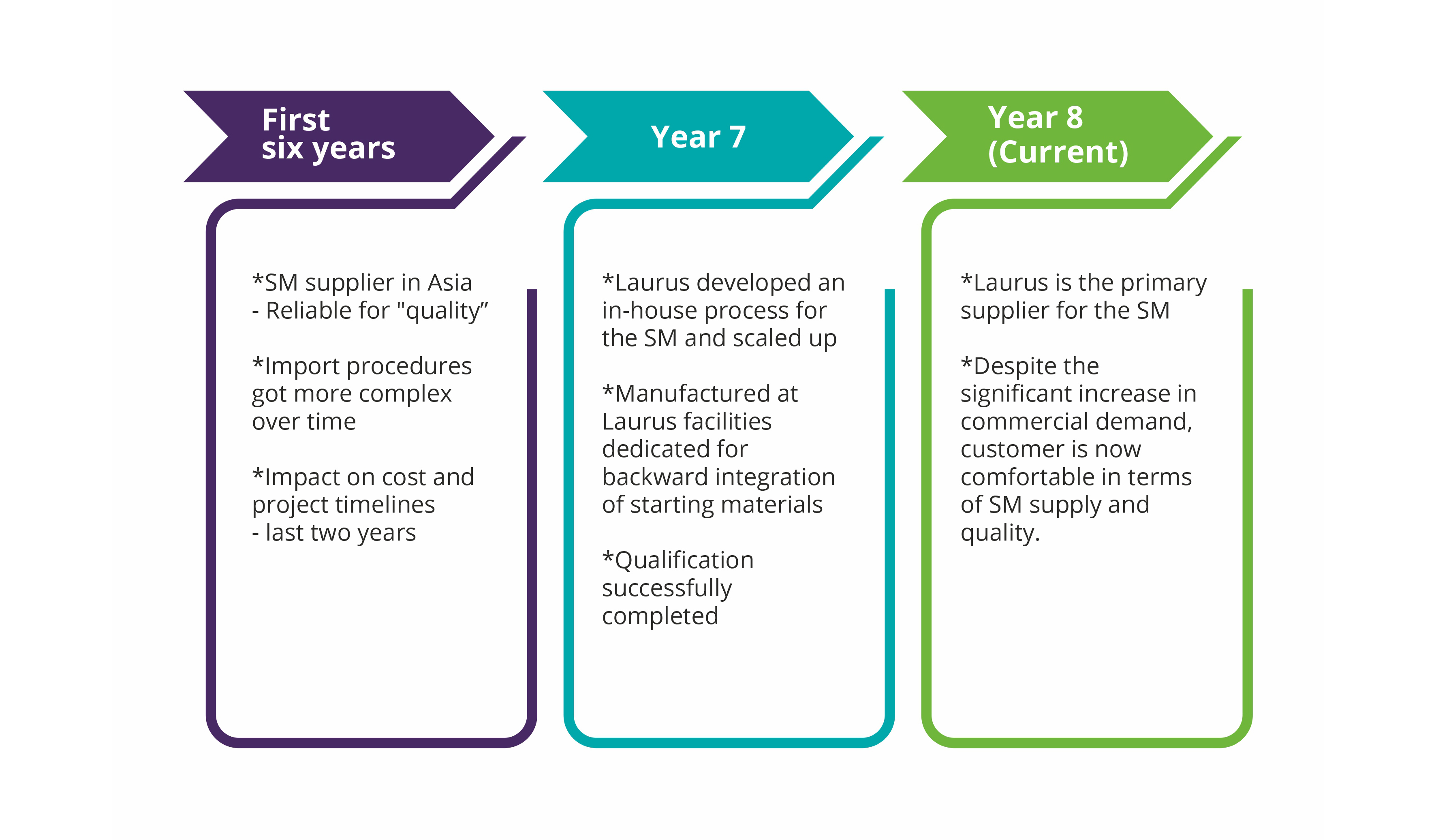
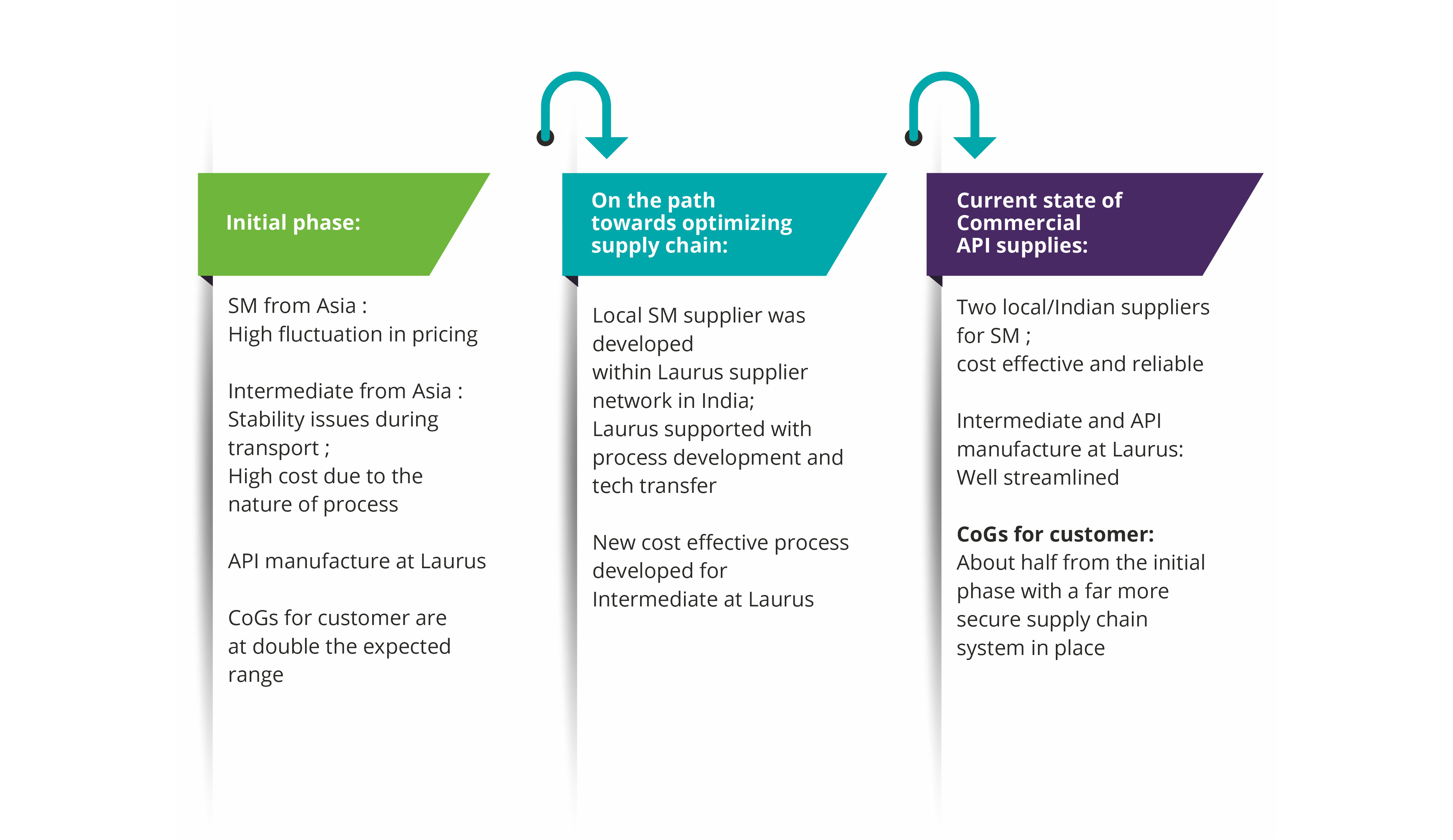
Laurus Synthesis has proven expertise in the manufacture of high-potent APIs and intermediates, at both pilot and commercial scale. Our dedicated high-potent cGMP manufacturing facilities can support small-scale synthesis as well as commercial-scale manufacturing. The facilities are designed to enable operational containment levels of Occupational Exposure Levels (OEL) <1mg /m3. In total, Laurus has a capacity of>300KL for high-potent drug substance manufacturing (Cytotoxic & steroidal hormones).
Key Features
Dedicated facility with isolated staging area for potent RMs to prevent cross-contamination
Operations under containment for dispensing potent RMs and isolation of potent products
Dedicated storage area in the containment facility (also for cold chain materials)
Best in class advanced packaging operations through isolators
Highly secure waste management processes in line with the guidelines
Fully contained powder processing areas (Sifter, Multi Mill & Micronizer)